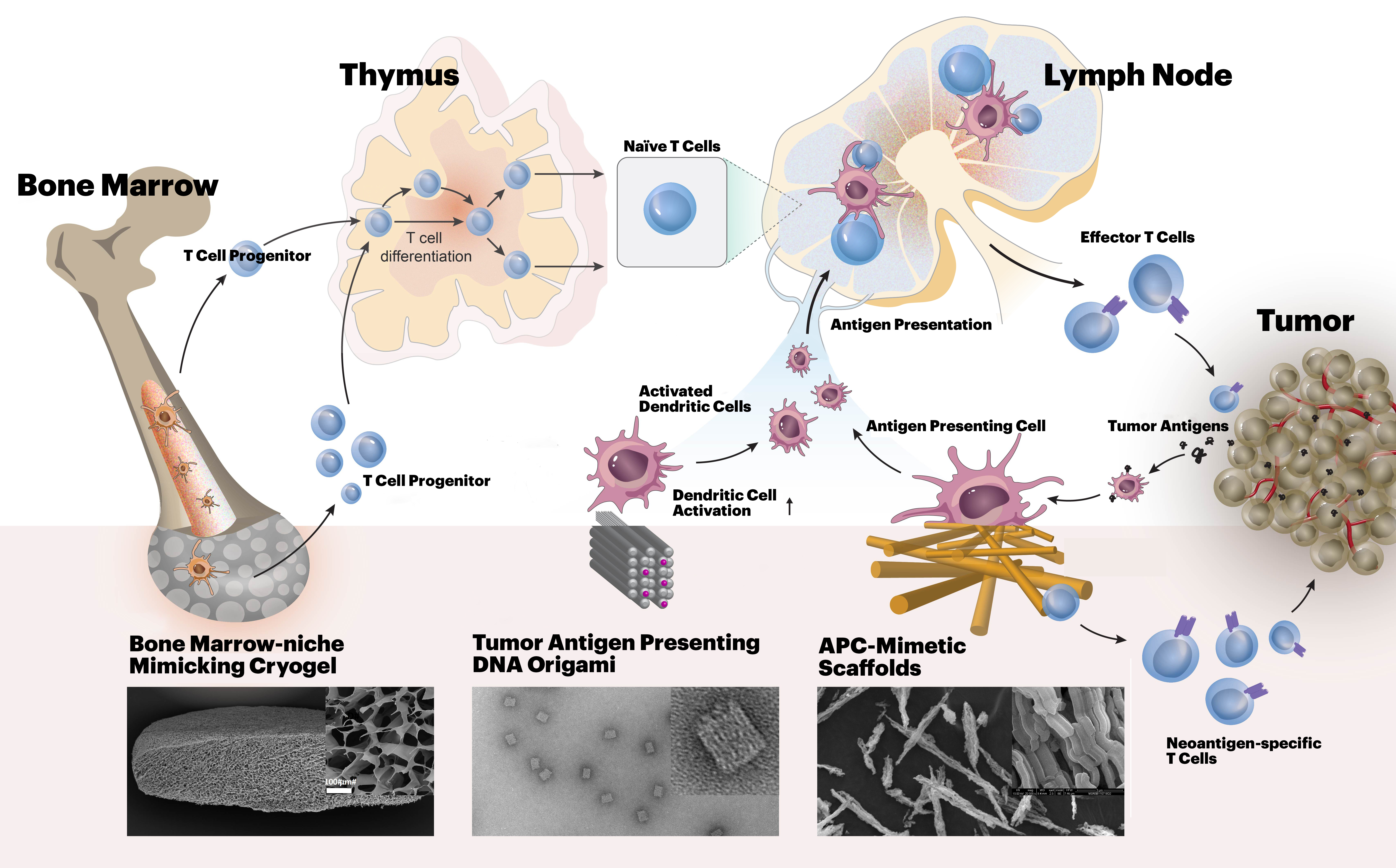
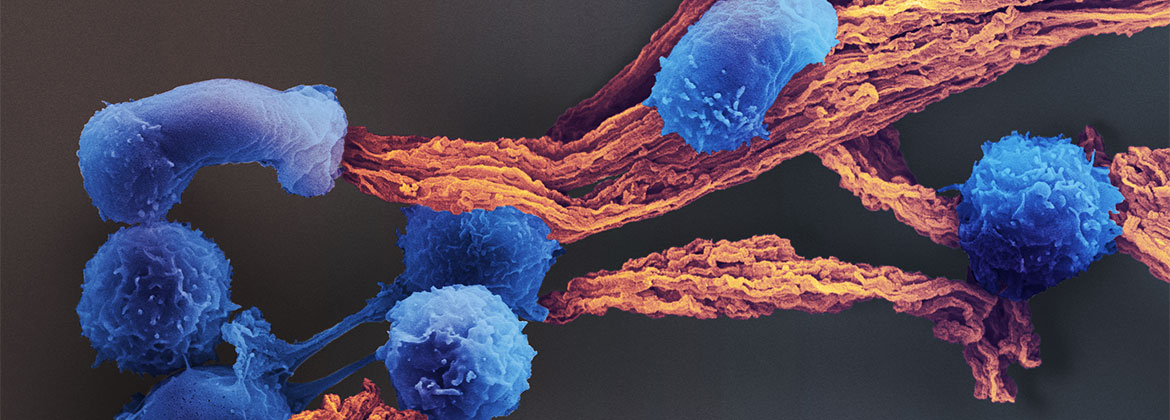
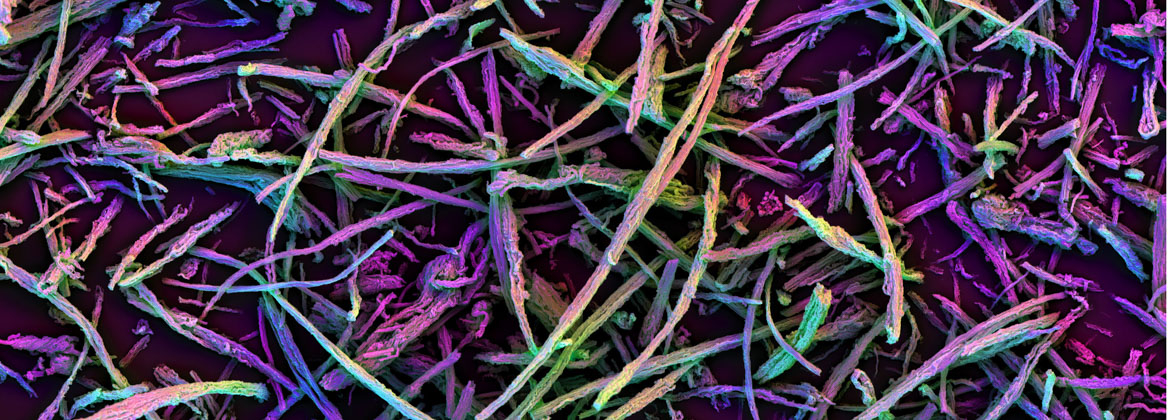
The mission of the i3 Center is to create biomaterials-based approaches to enable anti-cancer immune-therapy in settings where it currently is limited, such as myeloid malignancies and solid tumors.
The Harvard i3 Center is part of NIH’s Cancer Moonshot initiative that was formed to accelerate cancer research to make more therapies available to more patients, while also improving the ability to prevent cancer and detect it at an early stage. One component of the Moonshot initiative is to develop centers focused on Immune-Engineering to Improve Immunotherapy (i3 Centers).
T lymphocyte (T cell) responses are key to cancer immunotherapy, and this Center will create biomaterials to address a number of fundamental questions in immunoengineering related to T cell biology and cancer therapy. Our approach benefits from both a deep scientific understanding of the relevant immune/stem cell biology, an appreciation for the clinical realities of the motivating diseases and their treatments, and technical materials expertise in multiscale design of receptor, cell and tissue-interactive biomaterials, and experience in the implementation of novel cell-based therapeutics. For this purpose, we combine the expertise in bioengineering at the Wyss Institute for Biologically Inspired Engineering at Harvard University with the expertise in cancer immunology, oncology, hematology, biostatistics and stem cell biology at the Massachusetts General Hospital (MGH) and Dana-Farber Cancer Institute (DFCI).
Our groups have previously made major contributions to the development of checkpoint blockade therapy, neoantigen vaccines, cellular therapies, and therapeutic biomaterials, and have a long track record of highly productive collaborations. This i3 Center is expected to yield major scientific and translational advances in cancer immunotherapy.