By Benjamin Boettner

(BOSTON) – Harvard’s “Immuno-engineering to Improve Immunotherapy” (i3) Center at the Wyss Institute awarded cancer-immunologist Rizwan Romee, M.D. with a second annual grant. Romee and his group at the Dana-Farber Cancer Institute (DFCI) will develop natural killer (NK) cell-based therapies by leveraging biomaterials-based immune cell amplifying and educating approaches pioneered by David Mooney’s group at the Wyss Institute and the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS). This strategy could offer a new opportunity to advance immunotherapy to solid tumors, which thus far mostly have eluded innovative immunotherapies designed to turn patients’ immune system against cancer cells in their bodies.
With support from the National Institutes of Health (NIH) and its Cancer Moonshot initiative, the “Immuno-engineering to Improve Immunotherapy” (i3) Center was founded at Harvard University as a cross-disciplinary effort in which world-renowned immune-oncologists collaborate with bioengineers to broaden the reach of immunotherapies to solid tumors and blood cancers, such as multiple myeloma, that cannot be effectively treated yet.
Romee is Associate Professor of Medicine at Harvard Medical School and Director of the haploidentical donor transplant program at DFCI. He also leads the translational NK cell program at DFCI evaluating memory-like NK cells in combination with novel immune-modulatory agents in patients with advanced malignancies, including acute myeloid leukemia and myelodysplastic syndrome who relapsed after stem cell transplantation, multiple myeloma, and solid head and neck cancers.
“Rizwan Romee’s interests and therapeutic approaches around NK cells synergize beautifully with the i3 Center’s overall vision,” said David Mooney, Ph.D., who is a Founding Core Faculty member at the Wyss Institute, leads its Immuno-Materials Initiative, and also is the Robert P. Pinkas Family Professor of Bioengineering at the SEAS. “This new collaboration with him and his lab at Dana-Farber not only expands the center’s collaborative network, but could also significantly enhance NK-based approaches by integrating them with new biomaterials solutions, and thus potentially advance them toward patients who cannot benefit from the latest breakthroughs in immunotherapy.”
Adopt-a-Cell for cancer removal
Adoptive cell transfer (ACT) and specifically CAR T-cell therapies have generated tremendous excitement as immunotherapies. In CAR T-cell therapies, a patient’s own T cells, a type of immune cell that, in principle, can destroy cancer cells, are collected and then engineered in the laboratory to express a synthetic “chimeric antigen receptor” (CAR) on their surface. CARs enable the T-cells to bind to proteins known as tumor antigens on the surface of certain cancer cells, and help unleash T cells’ tumor cell-destroying potential. Following their expansion outside of the body and re-injection into the patient’s blood stream, CAR T-cells will search for and destroy the cancer cells.
The efficacy of CAR T-cells as “living drugs” in patients has generated tremendous excitement, and several CAR T-cell products have been approved by the U.S. Food and Drug Administration (FDA) to treat different blood cancers. However, CAR T-cells have proved far less effective in the treatment of solid tumors. Unlike blood cancer cells that float freely in circulating blood and are relatively accessible to various treatments, solid tumors create a microenvironment that forms a barrier to the movement of immune cells, and throttles the activity of those that manage to advance into the tumor core. Importantly, solid tumor cells also express fewer tumor-specific antigens that could be used to engineer CAR T-cells.
CAR T-cells also come with other challenges: although, in principle, they are a patient’s own cells and should be regarded as “self” by the body, as engineered versions they can induce significant toxicities, such as graft-versus-host disease, in which the transplanted cells attack the host, cytokine release syndrome, in which immune cells produce dangerous amounts of inflammatory molecules, as well as toxicities that damage the nervous system (neurotoxicities). In addition, immune cells can only be collected in small numbers from patients and need to be amplified ex vivo to generate an effective immunotherapy product. Researchers are working intensely to overcome all these challenges to CAR T-cell therapies, but succeeding in this might require entirely different approaches to the design of ACT therapies.
Naturally born cancer killers
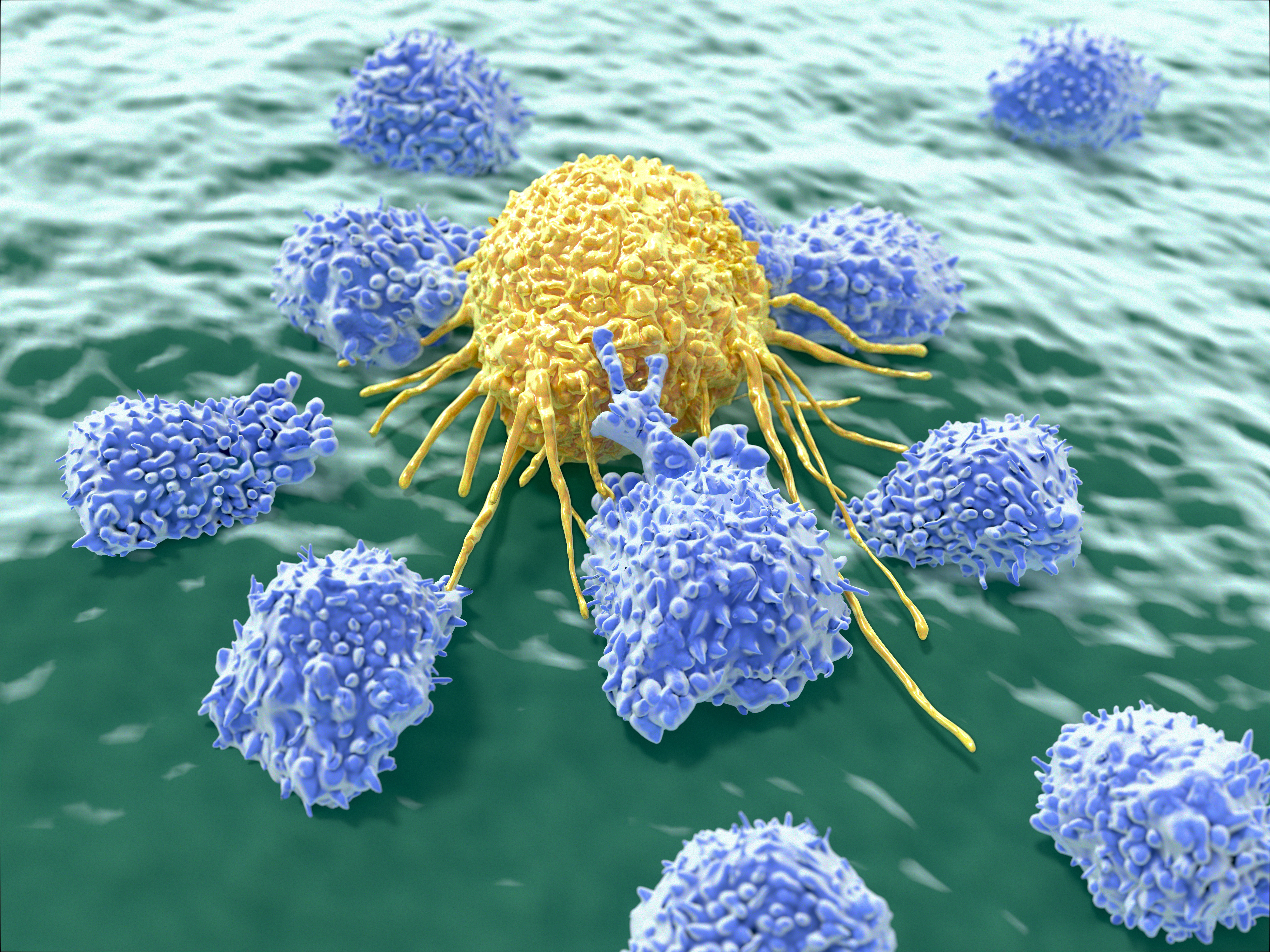
Romee’s interests and work under this grant are taking one such direction by harnessing a different type of immune cells known as natural killer (NK) cells that, unlike T cells, do not need to bind to specific tumor antigens in order to unleash their destructive potential, but rather respond to all cells that appear to be “non-self”. Although the general understanding of how NK cells develop and function is lagging behind that of T cells, their specific attack mode makes them interesting cells to be used in ACT-based therapies. Early clinical trials using NK cells have shown promise in cancer patients. Romee’s lab is working on several innovative projects aimed at genetically modifying NK cells to further enhance their tumor targeting abilities.
“NK cells are natural born killers that do not cause any of the toxicities that have been seen with CAR T-cells, and could bring us closer to the holy grail of adoptive cell therapy: the combination of efficacy, safety, and relative ease of supply,” said Romee. “In collaborating with David Mooney’s lab, with its strong expertise in immune cell-educating and amplifying biomaterials, we could find ways to activate and significantly increase the numbers of NK cells before re-injection into patients, which would make this approach much more viable, or even to keep activating and amplifying NK cells in vivo with implantable biomaterials following their re-injection.”
Thus far, NK cells collected from patients need to be expanded on layers of “feeder cells,” nourishing and activating cells that often have a cancerous origin. Feeder cells produce proteins known as cytokines, which enhance the multiplication of NK cells. But even using this laborious step, the resulting NK cell numbers are often limiting, and feeder cells that are carried over into ACT products are a risk to the health of patients.
By exposing NK cells to cell-permeable biomaterial scaffolds engineered to present and release some of the same cytokines, the researchers hope to overcome a central bottleneck in developing ACT as treatments for patients with solid tumors. Taking a step further, they also plan on developing implantable NK cell-homing scaffolds able to keep amplifying and activating NK cells after they have been injected back into patients. This approach could help extend the therapeutic efficacy of normal and genetically modified NK cells in patients.
For more information about the Harvard i3 Center: Biomaterials to Create T Cell Immunity, please contact Eileen Barrette.
PRESS CONTACT
Wyss Institute for Biologically Inspired Engineering at Harvard University
Benjamin Boettner, benjamin.boettner@wyss.harvard.edu, +1 617-432-8232
Immuno-engineering to Improve Immunotherapy (i3) Center
Eileen Barrette, mailto:eileen.barrette@wyss.harvad.edu

